Discover the DiagCor Difference



THE MOST TRUSTED BRAND
DiagCor is particularly concerned with quality and safety, and regularly participates in the national and international proficiency testing programs such as the American College of Pathology. Moreover, our paternity testing service has obtained ISO13485 certification.
- MLT sign-off
MLT sign-off makes sure that there are reliable results for customers
- Doctor referral
We require patients to seek doctors' professional recommendations before carrying out the tests for them
- Detailed report
We provide detailed reports with optimal number of markers to ensure the precision of lab data




- Biotech pioneer
1st independent private DNA laboratory in Hong Kong
- Research & Development
The one and only molecular diagnostic lab in Hong Kong with our own Research and Development team for technical support
- Advanced technique
Apply techniques such as polymerase chain reaction (PCR) and next-generation sequencing (NGS) to new testing procedures




- Significant representation
Hong Kong's largest biotech company in molecular diagnostics (MDx)
- Authorized & accredited
Granted numerous patents and ISO certifications
- Rich experiences
Analyzed over 500,000 clinical samples in our own laboratory with more than 10 years of hands-on experience in molecular diagnostics (MDx)




- Diverse backgrounds
More than 80 forefront scientists and leaders with expertise ranging from business knowledge to scientific specializations in the bioscience field
- In-depth knowledge
Extensive scientific knowledge with outstanding qualifications including Professors, Postdoctoral fellows, and PhD graduates from renowned institutions
- Prize-winning company
Won the prize of "the technology fast 500" awarded by Deloitte




Professional Services
After years of research and development experience, DiagCor Bioscience Inc. Ltd. had processed more than 500,000 samples, its products and services cover noninvasive prenatal testing, maternal blood Y-DNA detection, Y chromosome exclusion test (parent-child relationship test) genetic disease detection.
After years of research and development experience, DiagCor Bioscience Inc. Ltd. had processed more than 500,000 samples, its products and services cover noninvasive prenatal testing, maternal blood Y-DNA detection, Y chromosome exclusion test (parent-child relationship test) genetic disease detection.
Pregnancy Guide

重要聲明
詐騙警告
我司向來一直依法提供服務,對於近來市面上發現有達雅高公司的虛假偽造報告出現,香港達雅高生物科技有限公司現鄭重聲明如下:
達雅高生物科技有限公司在香港特別行政區的註册及營運地址為: 九龍新蒲崗 太子道東698號 寶光商業中心10樓。我們所有的化驗均有註册醫學化驗師跟進。
由達雅高所發出的化驗報告均可以透過官方網站驗證及下載,方便使用者分辨真偽。對此種涉嫌欺詐行為我們將通過法律手段給予嚴厲打擊,決不姑息。
現提醒患者必須保持警惕,不要接受假冒機構所提供的服務以造成不良後果,帶來經濟及精神損失。否則後果自負,達雅高絕不承擔任何責任。
現公佈達雅高官方聯繫方式如下:
官方網站: www.diagcor.com
報告下載網站:

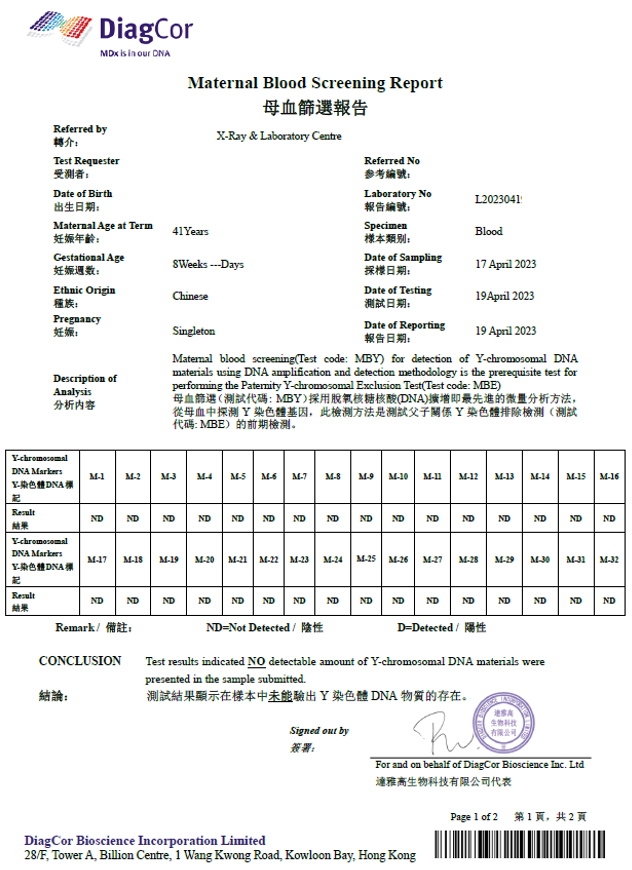
造假偽冒達雅高的報告
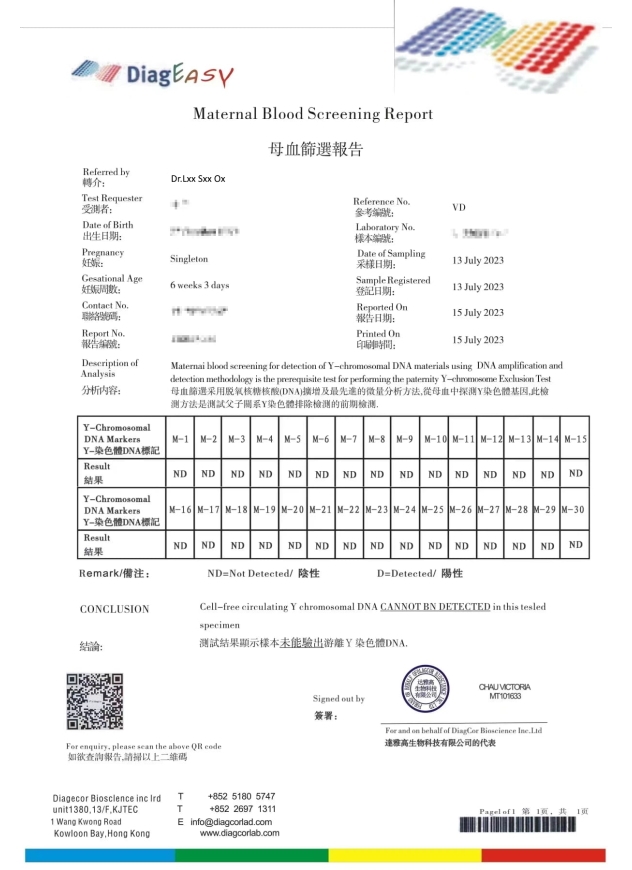
造假偽冒達雅高的報告